Major Characteristics for Medical Power Supply
Medical environments are known for its omnipresent risk for both vulnerable and healthy people. To ensure the utmost safety, the design of Medical Power Supplies is monitored by the strictest quality exigences and safety regulations.
As the heart of any electrical medical device, the medical power supplies must comply with the highest standards of performance, safety (IEC 60601) and reliability. Criteria such as MOPP, MOOP, low leakage current, EMC must be taken into consideration during the development stage.
As a result, Medical Power Supplies are equipped with higher levels of insulation and superior EMC performance, and we can distinguish one medically approved Power Supply from other electronics components by its exigent protection level, its high reliability and its extended life cycle of components.
MEAN WELL Medical Power Supplies
MEAN WELL is the global leading manufacturer of Standard Power Supplies in the market. With over 40 years of experience in the development and production of power supplies, MEAN WELL offers the widest range of high-quality Medical Power Supplies in 6 categories as below.
How to choose your Medical Power Supply?
As the keystone of one medical system, it is extremely important to select a safe and reliable Medical Power Supply. To choose the most suitable Medical Power Supply for the Medical system, the R&D engineers are strongly suggested to take, as guideline of selection, the listed criteria below into consideration
A. Electrical requirements
The input voltage range and required power should be defined for the application, as well as its output voltage and the needed current to power the application:
- Input voltage range: the most common input source is an AC source, and the range requirement is related to the destined market of the end devices. In the case of multiple market regions of the Medical system, it is strongly recommended to choose a Medical Power Supply with input voltage range covering at least the range of 100VAC to 240VAC.
- Power requirement: the required power depends mainly on the end device. It is suggested to keep in mind the derating caused by the unfavorable working temperature, as well as the potential peak current.
Output voltage and current: according to the design of the Medical system, some systems demand a constant current output while others need constant voltage. The R&D engineers should choose an adequate Medical Power Supply, with the right level and type of DC output, based on the design of the application.
B. Regulations
As each market territory and each application field imposes its own safety regulations on the end devices, it is crucial to understand which certifications are mandatory for the application and its target market(s) during the development process.
For instance, home healthcare devices are required to meet the standards of IEC 60601-1-11, while for equipment not directly in contact with the patient, IEC 62368 might still give one the possibility to get a medical certification for medical test and measurement equipment on a system level.
Meanwhile, each country adopts its specific safety standards for the application field (e.g. UL ANSI/AAMI ES60601-1 for the US, EN 60601-1 for EU countries, CAN/CSA-C22 3rd edition for Canada…etc.
C. Installation Method
The installation of the PSU can be either internally or externally depending on how the Power Supply is designed to be integrated into the system.
External solution:
Internal solution:
- Medical Enclosed type: PSU with plastic or metal case.
- Medical PCB type: A PCB populated with components which from the power supply. Normally this solution is without any mechanical protection such as a housing. For this reason, normally installed inside an application and not touchable by the user.
- Medical On board or Medical PCB mount: PSU designed to fit on a PCB. It can be either “open frame” or “encapsulated”. The second type is potted with compound to provide protection against external elements (e.g. moisture, corrosive elements, shock, vibration…etc.).
D. Other requirements
Medical systems are regulated with extremely vigorous exigences. On top of the above criteria, it is also important to verify that the other specific requirements are met:
- Class: Class I or class II
- Heat dissipation: Forced air cooling (with fan) or Passive cooling (without fan)
- Level of protection required: 2 x MOOP, 2 x MOPP
- Leakage current
- Overload protection: Constant Current Limiting or Hiccup mode
- Remote on/off function
- Current sharing function
- Parallel function
- …
Specific Requirements for Medical Power Supplies
I. IEC 60601-1:
Defined by the world known International Electrotechnical Commission (IEC), IEC 60601-1 is a series of technical standards destined to ensure the safety and performance of electrical equipment in the medical industry. On one hand, some digression from the IEC 60601-1 standards exists. For example, EN 60601-1 for Europe and ES 60601-1 for the US market which are harmonized with the IEC standards. One the other hand, there are also some “collateral” standards to IEC 60601-1; such as IEC 60601-1-2 which is the 4th edition of collateral EMC standards to IEC 60601-1.
II. MOOP and MOPP
In the medical environment, risk is constantly present for patient and/or operator of a medical device. Aiming to protect both groups from electrical medical devices, MOOP and MOPP are introduced to monitor the level of insulation.
- MOOP stands for Means of Operator Protection. This category is related to electronic devices which are handled by trained operators, and do not come into direct contact with the patient. The medical device with 2 x MOOP has an isolation of 3000V AC isolation.
- MOPP, on the other hand, is the Means of Patient Protection; all electronic devices with direct physical contact with patients must meet stricter standards and require a double isolation between input and output.
Presuming that patients are vulnerable; MOPP standards are specially designed to protect them from any potential electric shock. In the design, MOPP requires the medical devices to have two separate insulation barriers.
Therefore, devices are MOOP and MOPP classified depending on the type of contact with patients and operators.
| Classification | Required Isolation | Required Creepage | Requirement Insulation |
| MOOP | 1500V AC | 2.5mm | – |
| 2 x MOOP | 3000V AC | 5mm | Reinforced |
| MOPP | 1500V AC | 5mm | – |
| 2 x MOPP | 4000V AC | 8mm | Reinforced |
1 MOOP and MOPP under IEC60601-1 are different in the levels of isolation, creepage, insulation.
III. Low leakage current
The essential use of medical equipment is on the human body, therefore the chance and duration of contact with this equipment is higher and longer. Having electric current running throughout the body can be extremely dangerous and might even result in death. For example, a current as low as 40mA could already be fatal to a healthy person, while a weakened person’s tolerance is even lower.
To protect all people in contact with the devices from the electric shock, medical power supplies must meet strict requirements in terms of leakage current. While Applied Parts (AP) indicate the parts of a Medical device or a Medical system which, during normal use, come into direct physical contact with a patient. The standards of IEC60601-1 give a clear definition of the acceptable values of leakage current for each classification of Applied Parts (AP), and the classifications are further divided into 2 categories: “NC” for normal condition and “SFC” for Single fault condition.
Leakage Current |
Type B | Type BF | Type CF | |||
| NC | SFC | NC | SFC | NC | SFC | |
| Earth Leakage Current | 5mA | 10mA | 5mA | 10mA | 5mA | 10mA |
| Enclosure Leakage Current | 100µA | 500µA | 100µA | 500µA | 100µA | 500µA |
| Patient Leakage Current | 100µA | 500µA | 100µA | 500µA | 10µA | 50µA |
2 Different Leakage Current limits defined by IEC60601-1 according to the medical environment: Type B, Type BF, Type CF
IV. Classification of medical environment
Just like medical power supplies, end systems in medical environments are also regulated by strict isolation and leakage current requirements.
Depending on the type of physical contact between patient and medical device, there are three main classification types of Applied Parts (AP) in the medical environment: Type B, Type BF, Type CF.
- Type B (Body)
Devices with no direct physical contact with patients. Examples: medical bed, medical laser…etc. - Type BF (Body Float)
Devices with physical contact with patients and might present risk in the case of device failure. Examples: Incubators, diagnostic equipment, etc. - Type CF (Cardiac Float)
Direct contact to the patient’s heart, risk of injury or death in the event of device failure. Examples: Defibrillators, heart-lung machines, etc.
Note: Medical power supplies for type BF & CF devices are designed to meet 2 x MOPP
V. EMC standard and limits
Malfunction induced by electromagnetic or interference could be fatal when it comes to life-saving devices. The EMC standards EN 55011 for electromagnetic interference and IEC 60601-1-2 with reference and electromagnetic immunity must be considered.
The 4th edition of the IEC-60601-1 standard on EMC is much more vigorous with electromagnetic immunity than the previous edition. Medical devices must now be immune to HF fields up to 2.7GHz, which represent an increase by 0.2GHz. To prevent damage caused by electrostatic discharge, the limits have also been increased accordingly. For contact discharge, the level has been increased from 6 to 8kV. For air discharge, it has been increased from 8 to 15kV compared to the previous edition.
VI. EU Medical Device regulation (MDR)
The European Union Medical Device Regulation (MDR) published 2017, will soon replace the current Medical Device Directive (MDD) (93/42/EEC) and the EU’s Directive on active implantable medical devices (90/385/EEC). All development engineers and manufacturers related to Medical Devices within Europe will need to follow the Medical Device Regulation of 2017 published by European Parliament.
VII. ISO 13485
ISO 13485 is the standard for the quality management system for medical devices. The requirements are destined to organizations involved in the design, production, storage, installation and maintenance of medical devices and other related services.
Learn more about Medical Power Supplies
Explore our blog for insightful technical notes about Medical Power Applications.
Got questions?
Look at the section below to find the most frequently asked questions (with answers)
we received in Medical Power Applications.
The GTIN number can be found directly on the www.meanwell.com:

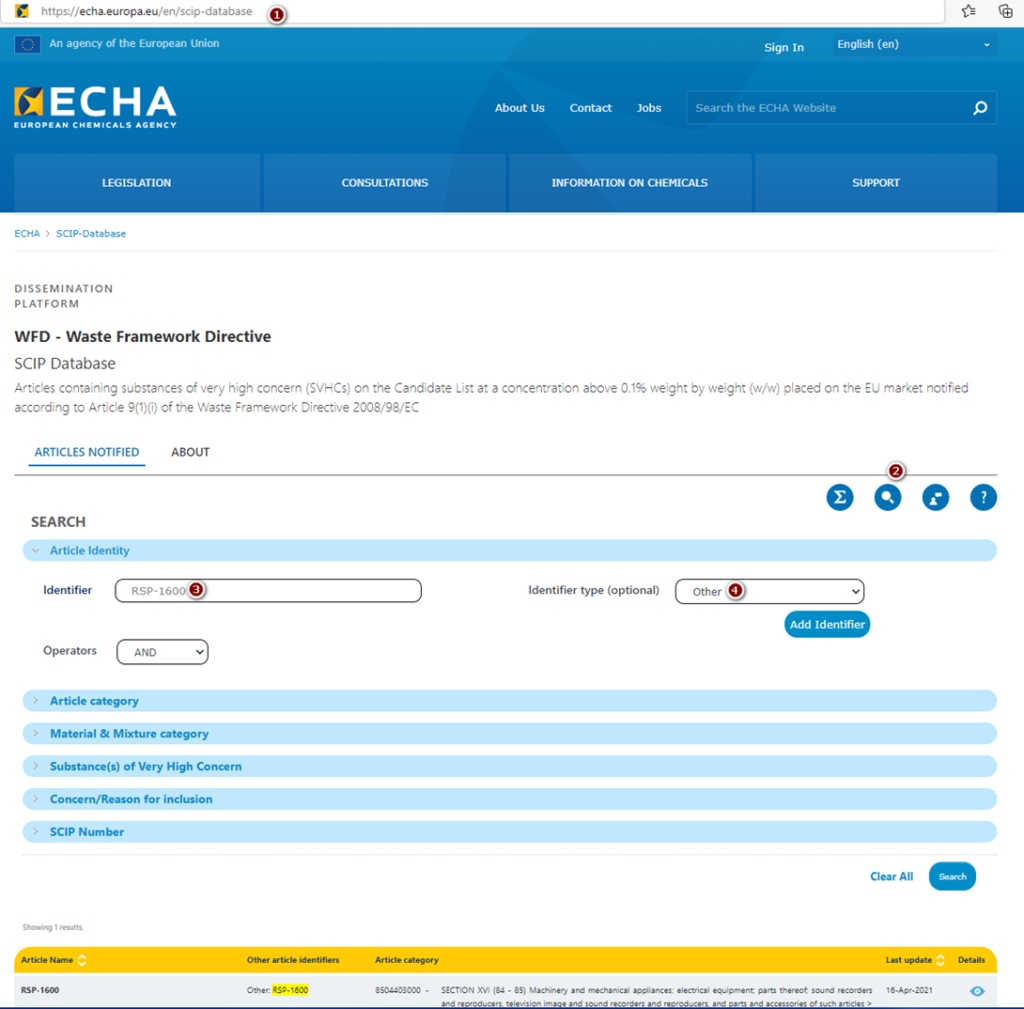
Yes, MEAN WELL products are registered in SCIP. To get such information for specific power supply, please follow the steps below:
- Go to https://echa.europa.eu/en/scip-database
- Under SEARCH option, choose „Article Identity” and write down model name e.g. RSP-1600.
- As “Identifier type (optional)”, please chose “Other”
- Click “Search” button
All the latest KNX firmware updates can be found on ETS Online Catalogue or MEAN WELL website dedicated to KNX products.
a. Got to KNX Building Automation Solution-MEAN WELL -> Downloads -> ETS Application Database
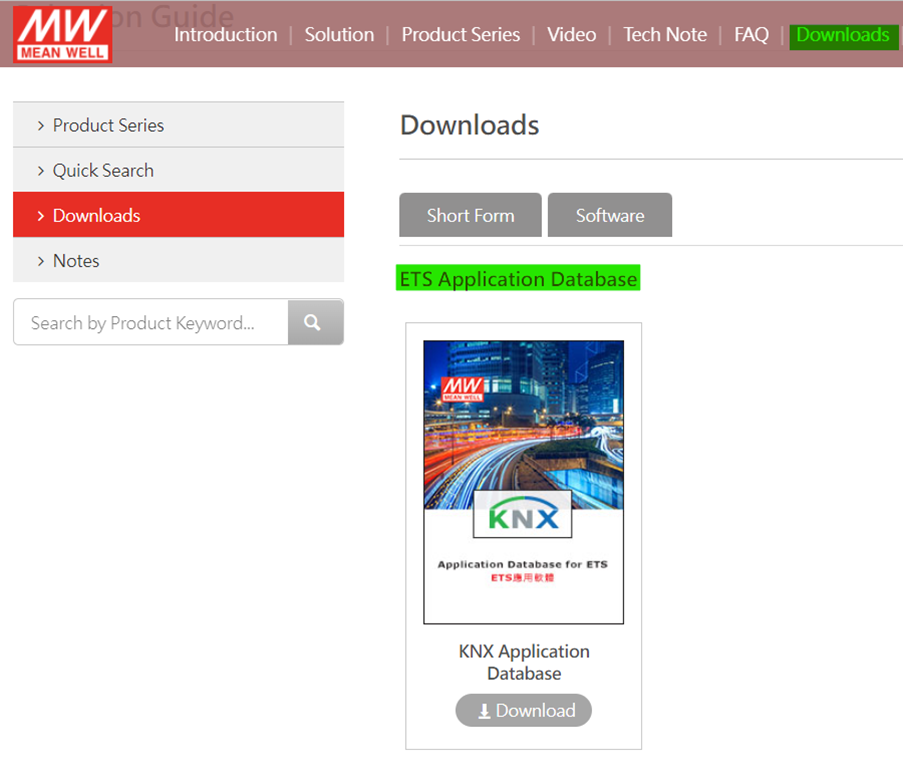
b. Click on Download button to see PDF file with all the hyperlink for specific products.
Find the latest news in the MEAN WELL APP

You can follow us on LinkedIn:

Go to:
https://www.linkedin.com/company/meanwell-europe-b-v-/
1. Click follow
And you could install our APP see our FAQ “How to subscribe to MEAN WELL’s newsletter?”
MEAN WELL’s distributor information can be found on Distributor Network-MEAN WELL Switching Power Supply Manufacturer
- Click on the region that you are located
- Select the country you are in
- Click on the search button
- Scroll down to see our local distribution channels
- Look for the distributor with a tick for the product group that you are looking for

MEAN WELL’s discontinued product schedule and End of Life products are normally updated 2 times per year, in January and in July and are published on www.meanwell.com. See FAQ “Where can I find MEAN WELL’s discontinued product schedule and End of Life information? “
The normal procedure for E.O.L. is:
- The product or series is announced in the Discontinued product list in January or July and announced as NRND (Not Recommend for New Design)
- 6 months later the lead time of the product or series will increase +30 days, the price will increase as well.
- Another 6 months later, the lead time will increase another +30 days (so + 60day compared to the original lead time), the price will increase again.
- Another 6 months later, the lead time will increase another +30day (so +90 days compared to the original lead time) and the price will increase again and additionally there will be a MOQ of 200pcs (and steps of 100 for higher quantities)
- After another 6 months the last buy is announced on the website. This will be the last opportunity to place an order for this product or series.
In total MEAN WELL’s End of Life, procedure will take 2 years. However, there are situations for instance that certification is expired, or some components can no longer be obtained by the market which will force to accelerate the EOL schedule. Therefore, it is always highly recommended from the moment that a product is on the discontinued list to design in one of our new generation products. If need any advice to this, please use the “Contact Us” function on this website.
MEAN WELL’s discontinued product schedule and End of Life products are normally updated 2 times per year, in January and in July. To see the full list, go to www.meanwell.com
1. Click on products
2. Click on Discontinued products for the schedule for the EOL schedule
Click on EOL for the MEAN WELL products which are End Of Life
You can use the “Contact Us” function on this website
MEAN WELL’s website provides you all the basic information about our Power Supplies. This includes company news, product announcements, ISO certificates, Specifications, test report, Certificates, ROHS declarations, Reach declarations and many more.
MEAN WELL’s products can be found on www.meanwell.com
1. Click on products and select the product category
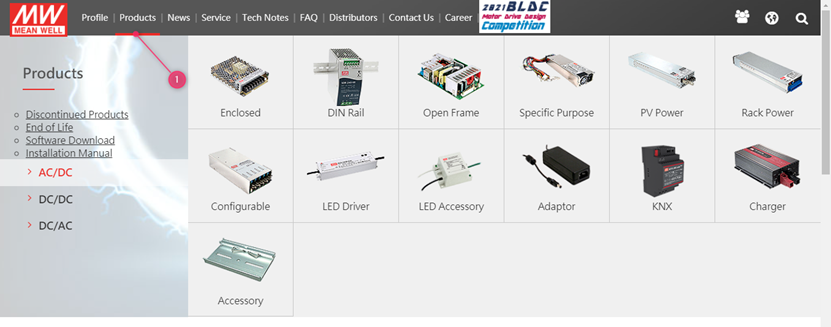
Or in case you already have a part number, you can use the search function on the website:
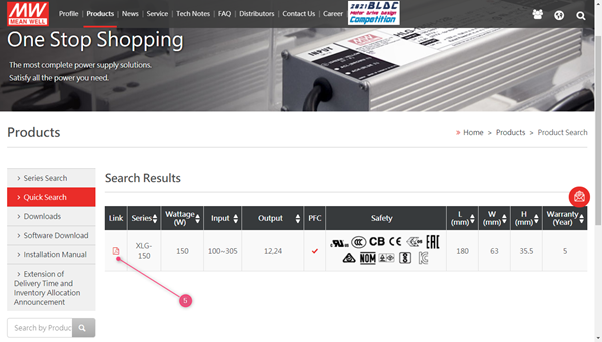
2. Use the search function on the website to find the right product
3. Fill in the series number in the search field (do not include the last extensions such as -12 in XLG-150-12)
4. Click the search button

5. Click on the PDF icon to open the specification
MEAN WELL has incorporated dust proofing and waterproofing into the majority of its LED power supply design. Mainly based on the international standard IEC60529, detailed descriptions can be found in the following table:
(Note: PSUs with IP64 rating or above are suitable for indoor or outdoor applications in sheltered locations)
IP xy protection level
| Degree of protection, foreign bodies (x) | Degree of protection, moisture(y) |
| 0. Not protected 1. Solid foreign object (>50mm) 2. Solid foreign object (>12mm) 3. Solid foreign object (>2.5mm) 4. Solid foreign objects of 1,0mm diameter and greater 5. Amount of dust that would interfere with normal operation Dust tight | 0. Not protected 1. Vertically falling water drop 2. Vertically falling water drop when enclosure is tilted up to 15 degrees 3. Water sprayed at an angle up to 60o on either side of the vertical 4. Water splashed against the component from any direction 5. Water projected in jets from any direction (12.5 liter/minute) 6. Water projected in powerfil jets from any direction (100 liter/minute) 7. Temporary immersion in water ( 1 meeter from the surface of the water for 30 minutes) 8. Continuous immersion in water, or as specified by the user / manufacture |
*IP64-IP66 level products are suitable for damp indoor or sheltered outdoor environment. For actual installation limitations, please refer to the corresponding IP level tests.
*All products cannot be continuously submerged in water.
*The definition of IP68 by MEAN WELL: Immerse a unit under test in 1 meter below the surface of the water, tested with a dynamic condition where 12-hour AC on; 12-hour AC off.
Test duration: 1 month.
LED Drivers are recommended operate at full load as long as it observes the working temperature specified in the datasheet, which means Tc measurement results should be equal to or less than the stated Tc in the datasheet. 5 years warranty complies as long as drivers operate within working Temperature and Tc. Limit as well.
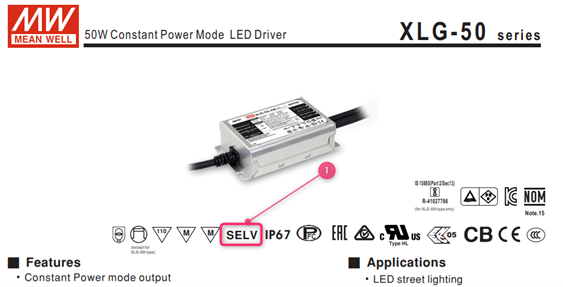
No, they are different. SELV means the LED driver will use a safety isolating transformer with double or reinforced insulation and the output voltage shall not exceed 120Vdc.
This is good for the end product safety certified if the LED driver with SELV output.
The definition of SELV was defined in the IEC 60950 standard but it is not defined in the IEC 62368 standard. This has been replaced with the ES1 Energy sources definition.
The definition of SELV is still applicable to the 61347-2-13 standard. In this standard it is that a LED driver will use a safety isolating transformer with double or reinforced insulation and the output voltage shall not exceed 120Vdc.
In the specification MEAN WELL’s 61347-2-13 certified LED drivers are marked with the SELV symbol in the case that the SELV requirements are fulfilled:
MEAN WELL’s safety reports, IEC reports and CB reports are not available online. In case you need these reports to validate your design with your certifying body, please contact your local MEAN WELL sales channel for support. If you are unable to get the support, please contact us via this website.
MEAN WELL’s User Manual can be found on www.meanwell.com
1. Go to products
2. Click on Installation Manual
3. Scroll down to find the user manuals for the different product families.

MEAN WELL’s Safety certifications can be found on www.meanwell.com
- Use the search function on the website
- Fill in the series number in the search field (do not include the last extentions suchs as -12 in XLG-150-12
- Click the search button

4. Click on the PDF Link
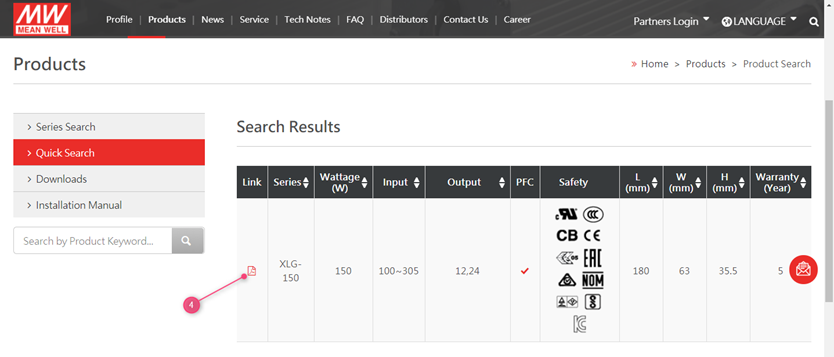
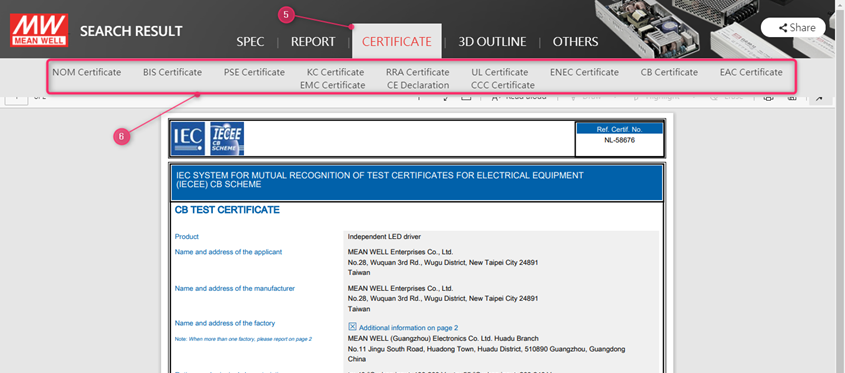
5. Click on the top on the certificate
6. All available certificates are shown and will show up once clicked upon
MEAN WELL’s CE declarations can be found on www.meanwell.com
- Use the search function on the website
- Fill in the series number in the search field ( do not include the last extentions suchs as -12
- Click the search button

4. Click on the PDF Link
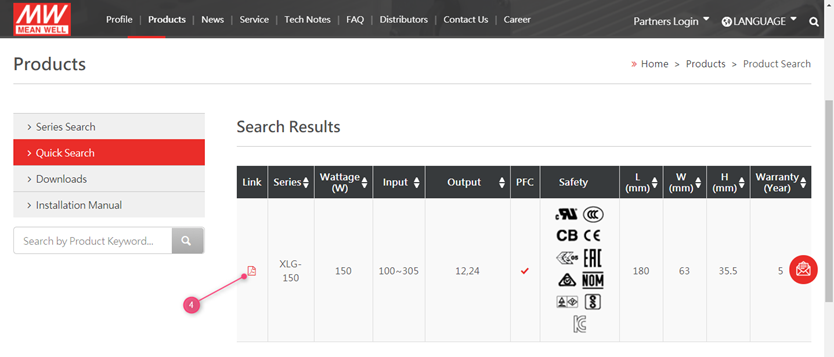
5. Click on the top on certificate
6. Click on CE declaration

Select (1) Products followed by (2) downloads
MEAN WELL’s EMI test guide can be found on www.meanwell.com
Select (1) Products followed by (2) Downloads
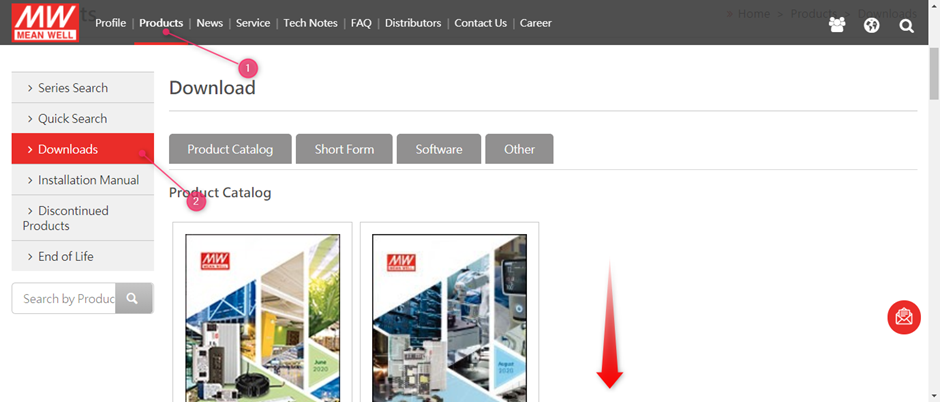
After this scroll down to find the EMI testing of Power guide
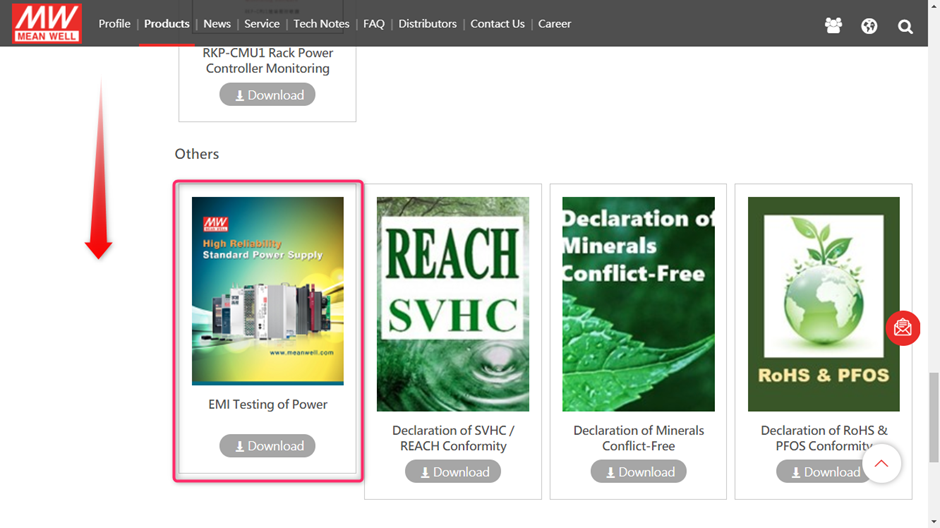
Or you can use this link to directly download the EMI testing guide:
EMI_statement_en.pdf
MEAN WELL’s RoHS and Reach statements can be found on www.meanwell.com
Select (1) Products followed by (2) Downloads:
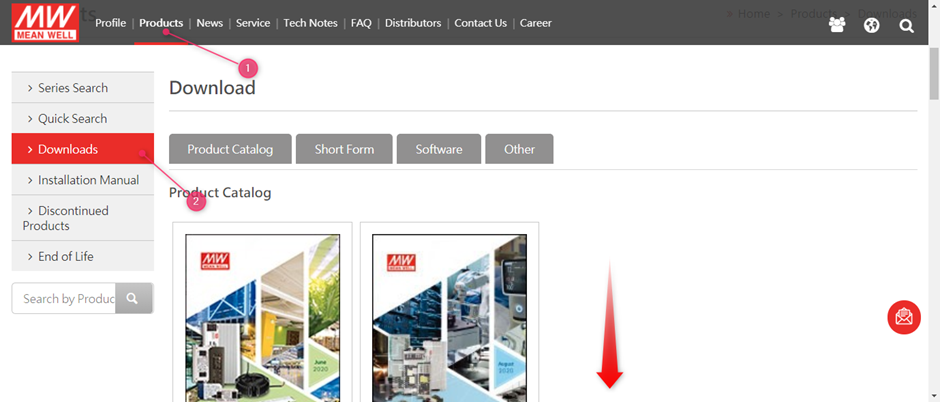
After this scroll down to find the RoHS declaration and Declaration of SVHC/ REACH conformity:
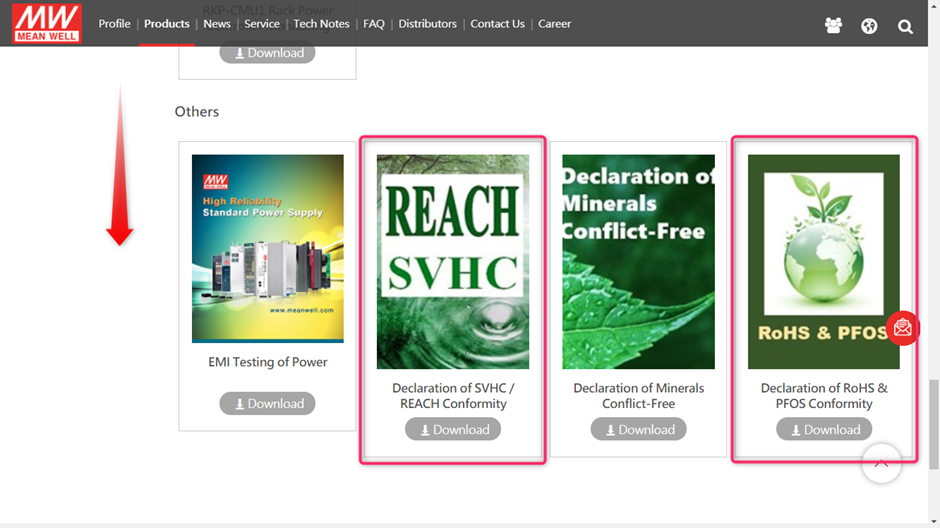
Or you can use the below links to download the declarations:
MEAN WELL’s Declaration of Conflict Free Minerals can be found on www.meanwell.com
Select (1) Products followed by (2) Downloads
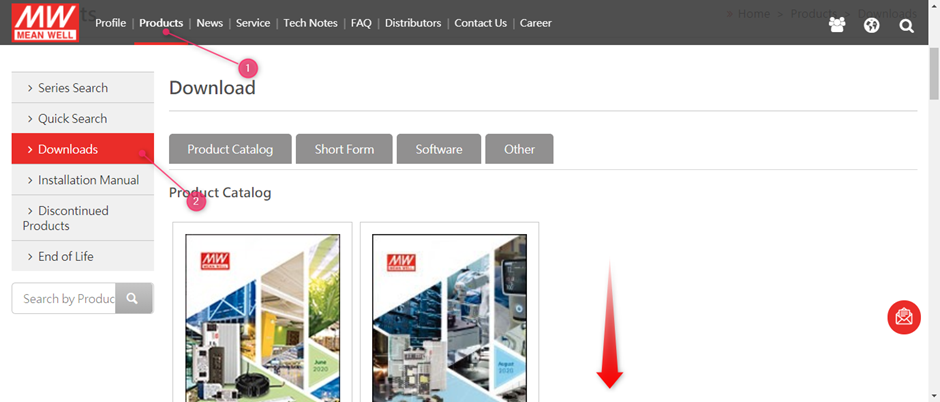
After this scroll down to find the Declaration of Minerals Conflict Free
Or you can use this Link to directly download the EMI testing guide: